Exploring the World of Gases: Charles's Law Calculator
Gases, with their unique properties and behaviors, play a crucial role in various scientific and industrial applications. Understanding and predicting the behavior of gases under different conditions is essential for researchers, engineers, and students alike. One fundamental gas law that aids in comprehending the relationship between temperature and volume is Charles's Law. In this article, we will delve into the principles of Charles's Law, its significance in the world of gases, and how a Charles's Law Calculator serves as a valuable tool for calculations and predictions.
Charles's Law: A Fundamental Gas Law
Charles's Law, named after the French scientist Jacques Charles, describes the relationship between the volume (V) and temperature (T) of a gas at constant pressure. The law states that, under constant pressure, the volume of a gas is directly proportional to its absolute temperature.
The mathematical representation of Charles's Law is:
V∝T
or
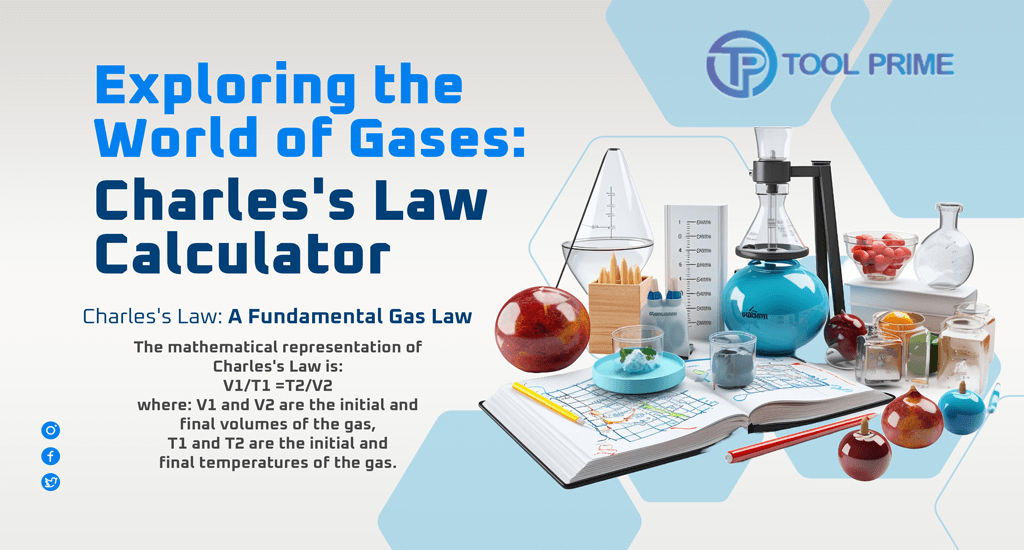
V1/T1 =T2/V2
where:
- V1 and V2 are the initial and final volumes of the gas,
- T1 and T2 are the initial and final temperatures of the gas.
This implies that as the temperature of a gas increases, its volume also increases proportionally, and as the temperature decreases, the volume decreases.
Understanding the Relationship
To better comprehend Charles's Law, let's consider a simple scenario. Imagine a gas confined in a container with a movable piston. If we heat the gas while keeping the piston in place, the gas molecules gain energy and move more rapidly. This increased molecular motion exerts pressure on the container walls, causing the volume of the gas to expand. Conversely, if we cool the gas, the molecules lose energy, move more slowly, and the volume of the gas decreases.
Charles's Law provides a quantitative expression of this observed relationship, allowing scientists and engineers to make predictions about the behavior of gases under varying temperature conditions.
The Role of Temperature in Charles's Law
An essential aspect of Charles's Law is the use of absolute temperature. Absolute temperature is measured in kelvins (K), and it starts from absolute zero, the point at which molecular motion theoretically ceases. The relationship between Celsius (°C) and kelvin is given by the equation:
T(K)=T(°C)+273.15
Using absolute temperature ensures that calculations are based on a scale where zero represents the absence of thermal motion.
This temperature-volume relationship is critical when working with gases, as it allows for consistent and accurate predictions regardless of the initial temperature scale used.
Charles's Law in Real-world Applications
The principles of Charles's Law find applications in various fields, influencing the design and operation of systems involving gases. Some real-world applications include:
1. Hot Air Balloons:
The expansion of air in a hot air balloon is governed by Charles's Law. By heating the air inside the balloon, its volume increases, causing the balloon to rise.
2. Gas Storage and Transportation:
Understanding how gases behave at different temperatures is crucial for storing and transporting them. For example, liquefied natural gas (LNG) is stored at extremely low temperatures to reduce its volume for efficient transportation.
3. Air Conditioning and Refrigeration:
The compression and expansion of gases in air conditioning and refrigeration systems are guided by Charles's Law. By manipulating the temperature and volume of gases, these systems can cool or heat spaces.
4. Automotive Engines:
Internal combustion engines rely on the expansion of gases to generate power. Charles's Law principles are integral in optimizing the efficiency of these engines.
5. Chemical Reactions:
In chemical reactions involving gases, temperature changes can influence the reaction rates and product yields. Charles's Law aids in predicting and controlling these reactions.
Challenges in Manual Calculations
While Charles's Law provides valuable insights into gas behavior, manually calculating the effects of temperature changes on gas volume can be complex and time-consuming. The need for accurate predictions in real-world applications makes the use of Charles's Law Calculator highly advantageous.
The Charles's Law Calculator: A Practical Solution
The Charles's Law Calculator is a digital tool designed to streamline calculations related to Charles's Law. It offers a convenient and efficient way to predict the behavior of gases under different temperature conditions without the need for complex manual calculations. Let's explore the key features and benefits of using a Charles's Law Calculator:
1. Instant Results:
Charles's Law Calculators provide immediate results, allowing users to obtain volume predictions quickly. This is particularly advantageous in scenarios where time is a critical factor.
2. Accuracy and Precision:
Digital calculators ensure a high level of accuracy and precision in calculations. This is essential in applications where small errors can have significant consequences.
3. Flexibility:
The calculator accommodates different units for temperature (Celsius or Kelvin) and volume, providing flexibility to users based on their preferences or the units used in specific scenarios.
4. User-friendly Interface:
Charles's Law Calculators typically feature a user-friendly interface, making it easy for individuals with varying levels of expertise to input values and obtain results.
5. Educational Tool:
For students learning about gas laws, the calculator serves as an educational tool, allowing them to experiment with different temperature and volume scenarios and observe the effects predicted by Charles's Law.
6. Accessible Anywhere:
Online Charles's Law Calculators are accessible from any device with an internet connection. This makes them a versatile tool that can be used in classrooms, laboratories, or on the field.
Using the Charles's Law Calculator: A Step-by-Step Guide
Using a Charles's Law Calculator is a straightforward process. Let's walk through a step-by-step guide on how to perform calculations using this tool:
Step 1: Access the Calculator
Visit the Charles's Law Calculator website or open the application on your device. Ensure you have an internet connection.
Step 2: Input Known Values
Identify the known values in your Charles's Law scenario. These would typically be the initial volume (V1), initial temperature (T1), and either the final volume (V2) or the final temperature (T2).
Step 3: Select Units
Choose the units for temperature (Celsius or Kelvin) and volume (e.g., liters, cubic meters) based on your preference or the units used in your scenario.
Step 4: Obtain Results
Once you've input the values, the calculator will instantly generate the result for the unknown variable (either V2 or T2). The result will be displayed clearly, making it easy to interpret.
Step 5: Experiment and Adjust
Take advantage of the calculator's flexibility to experiment with different scenarios. Adjust the known values and observe how the result changes, providing valuable insights into the temperature-volume relationship predicted by Charles's Law.
Step 6: Copy or Share Results
If needed, you can copy the results or share them through various means. This can be useful for documentation or collaboration purposes.
Real-world Scenarios:
Scenario 1:
Problem: A gas occupies a volume of 2.5 liters at a temperature of 300 K. What will be its volume if the temperature is increased to 400 K?
Solution: Input V1 =2.5 L, T1 =300 K, and T2 = 400 K into the calculator. The calculator will provide the result for V2, giving the predicted volume at the new temperature.
Scenario 2:
Problem: A researcher wants to study the effects of cooling gas from an initial temperature of 200°C to a final temperature of 100°C. If the initial volume is 3.0 liters, what will be the final volume?
Solution: Input
V1=3.0 L, T1=200°C, and T2=100°C into the calculator. The calculator will provide the result for V2, indicating the predicted volume at the final temperature.
Conclusion:
Charles's Law, with its insights into the temperature-volume relationship of gases, is a fundamental principle that finds applications in diverse fields. The Charles's Law Calculator emerges as a practical solution to the challenges associated with manual calculations, providing a quick, accurate, and user-friendly way to predict gas behavior under different temperature conditions.
Whether you're a student exploring gas laws in a classroom, a researcher studying the properties of gases, or an engineer designing systems that involve gas volumes, the Charles's Law Calculator serves as a valuable tool. Its accessibility, flexibility, and immediate results make it an indispensable resource for anyone dealing with the fascinating world of gases and their dynamic behaviors.